Properties of Matter Web quest
1. Find ten examples of chemical change
http://chemistry.about.com/od/matter/a/10-Chemical-Change-Examples.htm
1.
6.
2.
7.
3.
8.
4.
9.
5.
10.
2. Find ten examples of physical change
http://chemistry.about.com/od/matter/a/10-Physical-Change-Examples.htm
1.
6.
2.
7.
3.
8.
4.
9.
5.
10.
3. Describe the difference between a chemical and a physical change.
http://chemistry.about.com/od/lecturenotesl3/a/chemphyschanges.htm
Chemical change =
Physical Change =
4. List the properties of matter.
http://www.files.chem.vt.edu/RVGS/ACT/notes/Properties_of_Matter.html
1.
6.
2.
7.
3.
8.
4.
9.
5.
10.
5. Sketch the phases of matter from a solid to a gas, and describe each phase.
(Use the same number of atoms in each)
http://www.chem.ufl.edu/~itl/2045/lectures/lec_f.html
6. If you have the same amount of matter (atoms) which state of matter will
occupy the least amount of volume and which will occupy the most?
Analyze your sketches from the phases of matter.
http://www.chem.ufl.edu/~itl/2045/lectures/lec_f.html
7.
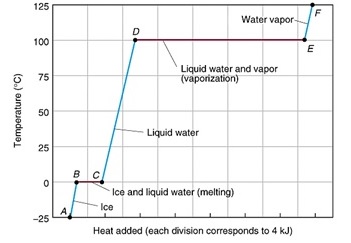
Analyze the graph above and determine the
Freezing point of water = _______
Boiling point of water = ________
Which phase take requires the greatest amount of energy?____________ Explain
why?__________________________________________________________________________________
Briefly describe the following as the atoms go through the three phases:
|
|
Melting
|
Boiling
|
Freezing
|
|
Distance between atoms
|
|
|
|
|
Volume occupied by the atoms
|
|
|
|
|
Density of atoms
|
|
|
|
|
Temperature of atoms
|
|
|
|
8. Plasma is the fourth phase, what is so special about the
mixture of neutral atoms, free electrons, and charged ions?
http://www.grc.nasa.gov/WWW/k-12/airplane/state.html
9. A liquid
and a gas are similar in they both…
http://www.grc.nasa.gov/WWW/k-12/airplane/state.html
10. A liquid and a gas are different because …
http://www.grc.nasa.gov/WWW/k-12/airplane/state.html
11. Based on your experience, which one can be compressed?
Gas or Liquid? Explain, and
give an example.
12. Define a mixture and
give two examples:
http://www.slideshare.net/tracyconover/mixtures-solutions-elements-compounds
Ex. 1.________________ Ex. 2.________________
13. Briefly describe a method to separate two substances in a mixture.
14. Briefly describe the difference between a Solute, Solvent, and a Solution.
15. 2 Examples of Solutions= 1._______________________2.______________________
16. Explain the difference between an atom and an element.
atom=
element=
17. Explain how a compound is different from a mixture.
18. Briefly describe the 6 types of chemical reactions
http://misterguch.brinkster.net/6typesofchemicalrxn.html
1.
2.
3.
4.
5.
6.
19. List the
evidence observed during a chemical reaction.
http://www.launc.tased.edu.au/online/sciences/PhysSci/pschem/change/Change5.htm
1._____________________2._______________________3._____________________4._____________________
5._____________________6._______________________
20. Which is the solvent and which is the solute when you mix Kool-Aid and
water? Explain
http://alex.state.al.us/lesson_view.php?id=29860
21. Take the quiz at
http://www.prepdog.org/9th/9scccpom1.htm and record your score =_______
