Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
Identify the type of reaction for the following
chemical equation:
CaCO3(s) ---> CaO(s) +
CO2(g)
a. | decomposition | c. | double replacement | b. | single replacement | d. | synthesis |
|
|
|
2.
|
Identify the type of reaction for the following
chemical equation:
2Na(s) + 2H2O(l) ---> 2NaOH(aq)
+ H2(g)
a. | single replacement | c. | double replacement | b. | synthesis | d. | decomposition |
|
|
|
3.
|
All of the following are acids EXCEPT
a. | vinegar | c. | soda pop | b. | ammonia | d. | lemon juice |
|
|
|
4.
|
Thanks to NASA, scientists have found an arsenic based microorganism living in
Mono Lake, California. For the rest of the organisms on Earth, they are all made up of carbon
based molecules called
a. | organic compounds | c. | inorganic compounds | b. | ionic bonds | d. | solvents |
|
|
|
5.
|
Boiled purple cabbage water will turn acids red and bases blue and green.
This type of liquid is called a(n)
a. | indicator | c. | catalyst | b. | inhibitor | d. | neutralizer |
|
|
|
6.
|
Which of the following is an example of a chemical change?
a. | Water freezing in winter into ice | c. | Burning a log of wood
| b. | Copper metal being drawn into wires | d. | Mixing salt and
water |
|
|
|
7.
|
A neutron has a
a. | positive charge | c. | negative charge | b. | neutral charge |
|
|
|
8.
|
Carbon is able to form covalent bonds to many other elements due to the number
of valence electrons it has
|
|
|
9.
|
An atom that has the same atomic number but a different number of neutrons
is called a(n)
a. | quark | c. | isotope | b. | ion | d. | gluon |
|
|
|
10.
|
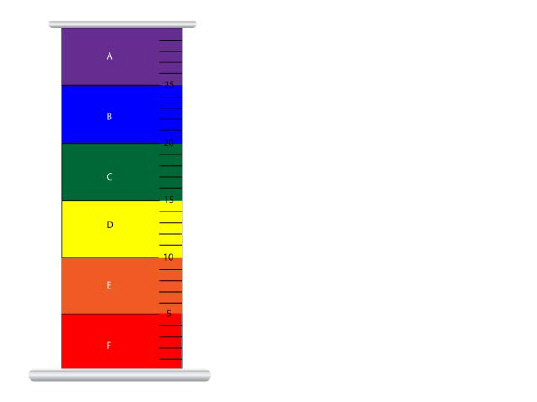 Place the liquids, contained in the
graduated cylinder, in order of most dense to least dense. a. | abcdef | c. | abefcd | b. | cbafed | d. | fedcba |
|
|
|
11.
|
You notice that your front lawn (Kentucky Bluegrass) is a light green color in
all areas except for the dark green spots where your dog has urinated. You take a pH reading of
the soil below the grass and it shows a pH of “8”.
a. | The soil contains too acidic | c. | The soil is neutral
| b. | The soil contains too basic |
|
|
|
12.
|
One substance is changed into two different substances
a. | physical change | c. | chemical change | b. | no
change |
|
|
|
13.
|
Identify the type of reaction for the following
chemical equation:
Mg(s) + H2O(g) ---> MgO(s)
+ H2(g)
a. | single replacement | c. | decomposition | b. | synthesis | d. | double
replacement |
|
|
|
14.
|
Which radiation type emits waves and does not form a new element?
a. | alpha | c. | gamma | b. | delta | d. | beta |
|
|
|
15.
|
The maximum number of electrons that can be filled in the first valence shell or
energy level is
|
|
|
16.
|
Which of the following is an isotope of an atom with 8 protons and 8
neutrons?
a. | 9 protons, 8 neutrons, 9 electrons | c. | 8 protons, 9 neutrons,
9electrons | b. | 16 protons, 16 neutrons, 8 electrons | d. | 8 protons, 8 neutrons, 8
electrons |
|
|
|
17.
|
Identify the type of reaction for the following
chemical equation:
Fe(s) + CuSO4(aq) ---> FeSO4(aq) +
Cu(s)
a. | double replacement | c. | synthesis | b. | decomposition | d. | single
replacement |
|
|
|
18.
|
Which solutions are in the correct order of acid to base?
a. | bleach, vinegar, water | c. | vinegar, water, bleach | b. | vinegar, bleach,
water | d. | water, vinegar,
bleach |
|
|
|
19.
|
Saturated hydrocarbons, such as gasoline or petroleum, contain carbon and
hydrogen. These bonds are
a. | single, double, and triple | c. | double (C=H)
only | b. | single (C-H) only | d. | Triple (C=H) |
|
|
|
20.
|
Identify the type of reaction for the following
chemical equation:
MgO(s) + H2O(l) --->
Mg(OH)2(s)
a. | combustion | c. | synthesis | b. | double replacement | d. | single
replacement |
|
|
|
21.
|
Identify the type of reaction for the following
chemical equation:
2KOH + H2SO4 ---> K2SO4 +
2H2O
a. | synthesis | c. | single replacement | b. | double replacement | d. | decomposition |
|
|
|
22.
|
All of the following are bases EXCEPT
a. | vinegar | c. | bleach | b. | ammonia | d. | soap |
|
|
|
23.
|
Hydrocarbons contain
a. | Hydrogen and Carbon | c. | Helium and Carbon | b. | Iron and Carbon | d. | Oxygen and
Carbon |
|
|
|
24.
|
If a stable atom has 3 protons and 4 neutrons how many electrons will it
have?
|
|
|
25.
|
Which of the following is an example of a physical change?
a. | A snowman melting in the Sun
| c. | Rusting
nail | b. | Frying an egg | d. | Moldy
cheese |
|
|
|
26.
|
All of the following are acids EXCEPT
|
|
|
27.
|
The Nobel gases do not react with other elements because
a. | they have multiple oxidation numbers | c. | there is only one branch on their
tree | b. | they need to gain electrons but not protons | d. | they have 8 or 2 valence
electrons |
|
|
|
28.
|
Identify the indicator below.
a. | purple cabbage | c. | lemon juice | b. | soap | d. | bleach |
|
|
|
29.
|
Which numbers balance the equation: ___H2+ ___O2 ---> ___ H2O
a. | 1,2,1 | c. | 1,1,1 | b. | 2,2,2 | d. | 2,1,2 |
|
|
|
30.
|
If a stable atom has 4 electrons and 5 neutrons, how many protons does it
have?
|
|
|
31.
|
The discovery of a nucleus and the empty space between the nucleus and the
electron shell ,using goold foil and radiation, was made by
a. | Newton | c. | Wegner | b. | Rutherford | d. | Einstein |
|
|
|
32.
|
If two atoms have opposite charges the atoms will
a. | magentize | c. | attract | b. | ionize | d. | repel |
|
|
|
33.
|
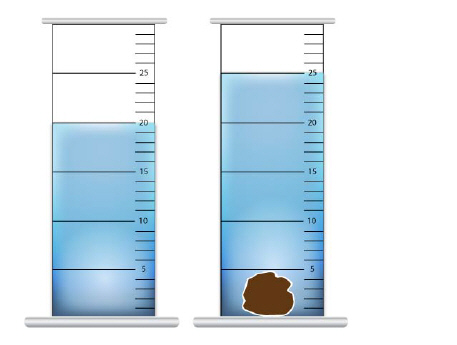 A student measures a rock to be 10 grams,
find the density of the rock by measuring the displacement of water before and after a rock is placed
in a graduated cylinder. a. | 2 g/ml | c. | .5 ml/g | b. | 5 g | d. | 50 gml |
|
|
|
34.
|
All of the following are examples of acids EXCEPT
a. | sour taste | c. | releases hydrogen ions | b. | releases hydroxide
ions | d. | corrosive |
|
|
|
35.
|
Measuring the force of gravity on a given mass is called
a. | density | c. | volume | b. | mass | d. | weight |
|
|
|
36.
|
Identify the type of reaction for the following
chemical equation:
2 Na(s) + Cl2(g) ---> 2NaCl(s)
a. | synthesis | c. | double replacement | b. | single replacement | d. | combustion |
|
|
|
37.
|
Anything that has mass and takes up space
a. | electrons | c. | matter | b. | protons | d. | temperature |
|
|
|
38.
|
A proton has a
a. | positive charge | c. | negative charge | b. | neutral charge |
|
|
|
39.
|
Which tool is used to detect radiation?
a. | Geiger counter | c. | Voltmeter | b. | pH indicator | d. | Ampmeter |
|
|
|
40.
|
An electron has a
a. | negative charge | c. | positive charge | b. | neutral charge |
|
|
|
41.
|
If two atoms have a negative charge the atoms will
a. | ionize | c. | attract | b. | repel | d. | magnetize |
|
|
|
42.
|
Carbon forms many different molecules in nature, this is due to the ability to
form covalent bonds because it has
a. | 4 valence electrons | c. | 2 valence electrons | b. | 12 valence electrons | d. | 6 valence
electrons |
|
|
|
43.
|
Identify the base.
|
|
|
44.
|
The subatomic particles found in a nucleus are
a. | neutrons and protons | c. | protons and electrons | b. | neutrons | d. | neutrons and electrons |
|
|
|
45.
|
The maximum number of electrons that can be filled in the second valence shell
or energy level is
|
|
|
46.
|
The amount of matter in an object is called
a. | density | c. | mass | b. | volume | d. | weight |
|
|
|
47.
|
Atomic number represents the number of
a. | quarks | c. | neutrons | b. | gluons | d. | protons |
|
|
|
48.
|
Identify the correct valence shell configuration for the first three energy
levels for Nitrogen (N)
a. | 0, 2, 5 | c. | 2, 2, 3 | b. | 2, 5, 0 | d. | 1, 2, 4 |
|
|
|
49.
|
A sour tasting candy with a pH of 2 is most likely
a. | acidic | c. | neutral | b. | basic |
|
|
|
50.
|
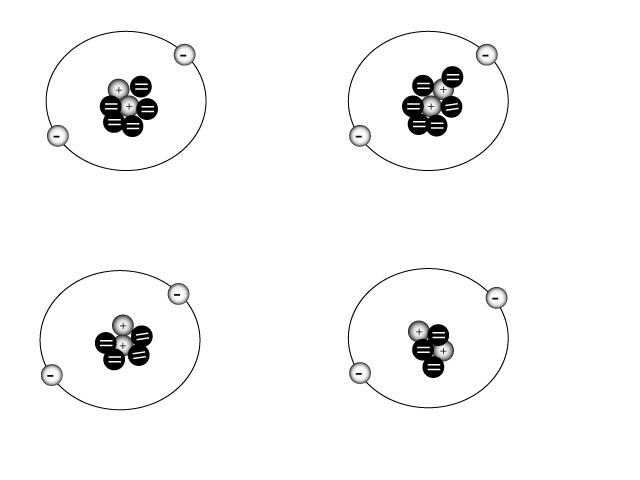 All of the diagrams above are an example of
a. | helium isotopes | c. | Helium ions | b. | hydrogen ions | d. | carbon atoms |
|
|
|
51.
|
Every carbon based life form dies and leaves Carbon-14 to decay into
Nitrogen-14. The radiation emitted from this process is called a(n)
a. | quark | c. | alpha particle | b. | beta particle | d. | gamma particle |
|
|
|
52.
|
To change a hydrangea flower bloom to a pink color the soil must be a pH of 6.0
- 6.2, which of the following would you add to a soil that has a neutral soil?  a. | soda pop | c. | soap | b. | ammonia | d. | toothpaste |
|
|
|
53.
|
Atomic mass is
a. | protons + neutrons | c. | protons | b. | neutrons | d. | protons +
electrons |
|
|
|
54.
|
A substance changes size, shape, and how fast the atoms vibrate.
a. | chemical change | c. | no
change | b. | physical change |
|
|
|
55.
|
As the elements move from left to right on the periodic table the protons
a. | is random | c. | increases | b. | decreases | d. | stays the same |
|
|
|
56.
|
Which of the following is an example of a chemical change?
a. | a piece of metal rusting | c. | Dew
drops condensing on a leaf at dawn | b. | A piece of rock
heating up in the Sun | d. | Ice melting to form
water |
|
|
|
57.
|
Identify the acid.
a. | Ca(OH)2 | c. | NH4OH | b. | HCl | d. | Mg(OH)2 |
|
|
|
58.
|
All of the following are bases EXCEPT
a. | HCl | c. | Ca(OH)2 | b. | Mg(OH)2 | d. | NH4OH |
|
|
|
59.
|
Trout fish can handle a pH range of about 6.5 to 5.0, if you found dead trout
floating in a pond and the pH is tested at a pH of 3. The pond is
|
|
|
60.
|
Which example is a base?
a. | vinegar | c. | soda pop | b. | ammonia | d. | lemon juice |
|
|
|
61.
|
The night before a sports event we load up with sugars called _____________ to
give us enough energy to compete the next day.
a. | lipids | c. | carbohydrates | b. | proteins |
|
|
|
62.
|
Identify the type of reaction for the following
chemical equation:
2NaCl + H2SO4 ---> Na2SO4 +2
HCl
a. | single replacement | c. | decomposition | b. | synthesis | d. | double
replacement |
|
|
|
63.
|
Which example is a property of a base?
a. | sour taste | c. | pH 1 - 6 | b. | releases hydroxide ions | d. | releases hydrogen
ions |
|
|
|
64.
|
Cannot be destroyed or created, only changed into a different
configuration
a. | mixture | c. | compound | b. | matter |
|
|
|
65.
|
Identify the type of reaction for the following
chemical equation:
Cl2(g) + 2NaBr(aq) ---> 2NaCl(aq) +
Br2(l)
a. | double replacement | c. | synthesis | b. | single replacement | d. | decomposition |
|
|
|
66.
|
If you mixed vinegar with baking soda the result is a pH change to
|
|
|
67.
|
The resistance of any physical object to a change
in its state of motion or rest
a. | mass | c. | acceleration | b. | velocity | d. | inertia |
|
|
|
68.
|
Identify the type of reaction for the following
chemical equation:
2Mg(s) + O2(g) ---> 2MgO(s)
a. | double replacement | c. | synthesis | b. | single replacement | d. | combustion |
|
|
|
69.
|
One acid in the human body is called an amino acid, these acids make up
a. | glucose | c. | lipids | b. | proteins | d. | carbohydrates |
|
|
|
70.
|
If a stable atom has an atomic mass of 10 and 5 protons how many neutrons will
it have?
|
Matching
|
|
|
Match the following a. | nuclear fusion | c. | Coal
burning | b. | nuclear fission |
|
|
|
71.
|
Used to produce most of the electricity through out the United States
|
|
|
72.
|
used to produce nuclear energy
|
|
|
73.
|
combines elements lighter than iron
|
|
|
74.
|
occurs in the Sun and in nuclear bombs
|
|
|
75.
|
divides elements heavier than iron
|
|
|
76.
|
emits large amounts of greenhouse gases
|
|
|
Match the following with the correct element a. | 18 protons, 22
neutrons | d. | 30 protons, 35 neutrons | b. | 3 protons, 4 neutrons | e. | 6 protons, 6 neutrons | c. | 16 protons, 16
neutrons |
|
|
|
77.
|
Zinc
|
|
|
78.
|
Carbon
|
|
|
79.
|
Argon
|
|
|
80.
|
Sulfur
|
|
|
81.
|
Lithium
|
|
|
Match the group with the correct oxidation number a. | Group 1, Alkali Metals | e. | Group 5A, Nitrogen group | b. | Group 2, Alkaline
Earth Metals | f. | Group 6A,
Oxygen group | c. | Group 3A, Boron group | g. | Group 7A, Halogen group | d. | Group 4A, Carbon group | h. | Group 8A, Nobel Gases
Group |
|
|
|
82.
|
Neutral or 0 oxidation
|
|
|
83.
|
+3
|
|
|
84.
|
-3
|
|
|
85.
|
-1
|
|
|
86.
|
-2
|
|
|
87.
|
-4 or +4
|
|
|
88.
|
+2
|
|
|
89.
|
+1
|
|
|
Match the atomic number with the correct element a. | Atomic number 1 | e. | Atomic number 20 | b. | Atomic number 5 | f. | Atomic number 25 | c. | Atomic number
10 | g. | Atomic number
30 | d. | Atomic number 15 | h. | Atomic number 35 |
|
|
|
90.
|
Neon
|
|
|
91.
|
Bromine
|
|
|
92.
|
Phosphorus
|
|
|
93.
|
Boron
|
|
|
94.
|
Hydrogen
|
|
|
95.
|
Calcium
|
|
|
96.
|
Manganese
|
|
|
97.
|
Zinc
|
|
|
a. | Acid | c. | Acid and Bases | b. | Base |
|
|
|
98.
|
Has a bitter taste
|
|
|
99.
|
Contains H+ (Hydrogen ions) when dissolved in water
|
|
|
100.
|
Turns red litmus paper to blue
|
|
|
101.
|
Conducts electricity
|
|
|
102.
|
reacts with metals to form Hydrogen gas
|
|
|
103.
|
Soapy feel
|
|
|
104.
|
Turns blue litmus paper to red
|
|
|
105.
|
Greater than 7 on the pH scale
|
|
|
106.
|
Contains OH- (Hydroxide ions)
|
|
|
107.
|
Sour taste
|
|
|
108.
|
Corrosive - will burn skin
|
|
|
109.
|
Less than 7 on the pH scale
|
|
|
Match the following symbols with the correct element a. | Ca | e. | Li | b. | Fe | f. | Ne | c. | C | g. | W | d. | O | h. | N |
|
|
|
110.
|
Calcium
|
|
|
111.
|
Carbon
|
|
|
112.
|
Tungsten
|
|
|
113.
|
Nitrogen
|
|
|
114.
|
Lithium
|
|
|
115.
|
Iron
|
|
|
116.
|
Oxygen
|
|
|
Match the following with the correct element a. | Group 1, period 1 | e. | Halogen, period
4 | b. | Group 2, period 4 | f. | Alkali metal, period 4 | c. | Nobel Gas, period 3 | g. | Alkaline earth metal, period
6 | d. | Metalloid, period 2 |
|
|
|
117.
|
Boron
|
|
|
118.
|
Argon
|
|
|
119.
|
Bromine
|
|
|
120.
|
Potassium
|
|
|
121.
|
Barium
|
|
|
122.
|
Calcium
|
|
|
123.
|
Hydrogen
|
|
|
Match the following a. | Strong acid | d. | Weak
base | b. | Weak acid | e. | Strong base | c. | Neutral |
|
|
|
124.
|
pH 7
|
|
|
125.
|
pH 8
|
|
|
126.
|
pH 14
|
|
|
127.
|
pH 1
|
|
|
128.
|
pH 6
|
|
|
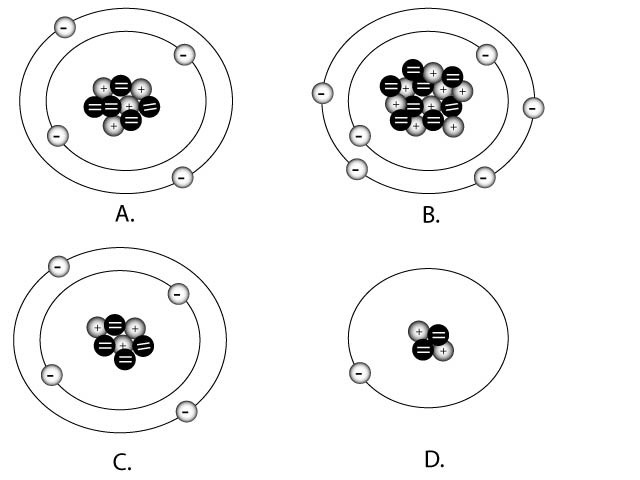 Match the correct charges with the diagrams
above
|
|
|
129.
|
negative 1 or -1
|
|
|
130.
|
positive 2 or +2
|
|
|
131.
|
neutral or = charge
|
|
|
132.
|
Positive 1 or +1
|
|
|
Match the states of matter according to molecular spacing and free
electrons.
|
|
|
133.
|
plasma
|
|
|
134.
|
gas
|
|
|
135.
|
solid
|
|
|
136.
|
liquid
|
|
|
Match the following change of states a. | melting | d. | condensation | b. | evaporation | e. | sublimation | c. | freezing |
|
|
|
137.
|
gas to a liquid
|
|
|
138.
|
solid to a gas
|
|
|
139.
|
solid to liquid
|
|
|
140.
|
liquid to a solid
|
|
|
141.
|
liquid to a gas
|
|
|
Match the physical properties of matter a. | ability to conduct heat | d. | solids dissolve in a
liquid | b. | solid, liquid, gas, plasma | e. | the ability of a
material to be elongated in tension | c. | the amount of mass in a given
volume | f. | capable of being extended or shaped by beating with a
hammer |
|
|
|
142.
|
density
|
|
|
143.
|
malleability
|
|
|
144.
|
thermal conductivity
|
|
|
145.
|
states of matter
|
|
|
146.
|
ductility
|
|
|
147.
|
solubility
|
|
|
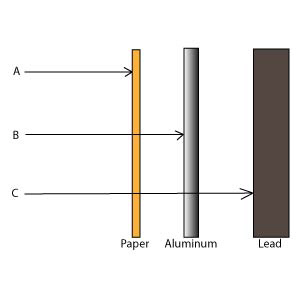 Match the radiation type with the correct
penetration above a. | “A” will not penetrate paper | c. | “C”
will not penetrate lead | b. | “B” will not penetrate aluminum
foil |
|
|
|
148.
|
Alpha
|
|
|
149.
|
Gamma
|
|
|
150.
|
Beta
|


