Matching
|
|
|
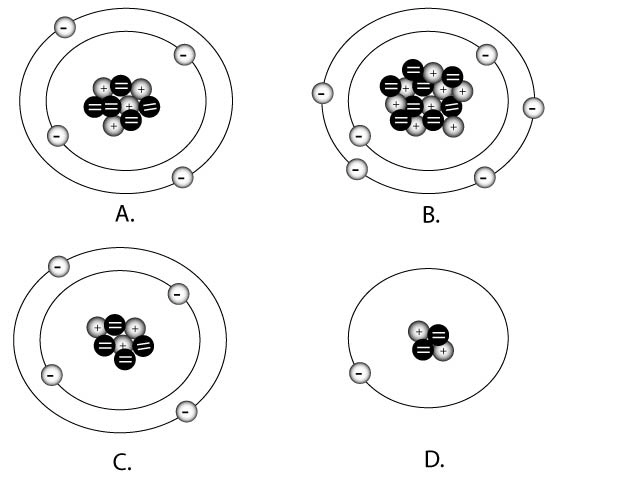 Match the correct charges with the diagrams
above
|
|
|
1.
|
neutral or = charge
|
|
|
2.
|
positive 2 or +2
|
|
|
3.
|
Positive 1 or +1
|
|
|
4.
|
negative 1 or -1
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
5.
|
If a stable atom has 4 electrons and 5 neutrons, how many protons does it
have?
|
|
|
6.
|
The discovery of a nucleus and the empty space between the nucleus and the
electron shell ,using goold foil and radiation, was made by
a. | Einstein | c. | Rutherford | b. | Newton | d. | Wegner |
|
|
|
7.
|
Atomic mass is
a. | protons + neutrons | c. | protons + electrons | b. | protons | d. | neutrons |
|
|
|
8.
|
A neutron has a
a. | neutral charge | c. | negative charge | b. | positive charge |
|
|
|
9.
|
If a stable atom has an atomic mass of 10 and 5 protons how many neutrons will
it have?
|
|
|
10.
|
A proton has a
a. | negative charge | c. | neutral charge | b. | positive charge |
|
|
|
11.
|
Which of the following is an isotope of an atom with 8 protons and 8
neutrons?
a. | 16 protons, 16 neutrons, 8 electrons | c. | 9 protons, 8 neutrons, 9
electrons | b. | 8 protons, 9 neutrons, 9electrons | d. | 8 protons, 8 neutrons, 8
electrons |
|
|
|
12.
|
An atom that has the same atomic number but a different number of neutrons
is called a(n)
a. | ion | c. | quark | b. | gluon | d. | isotope |
|
|
|
13.
|
The subatomic particles found in a nucleus are
a. | neutrons | c. | protons and electrons | b. | neutrons and
protons | d. | neutrons and
electrons |
|
|
|
14.
|
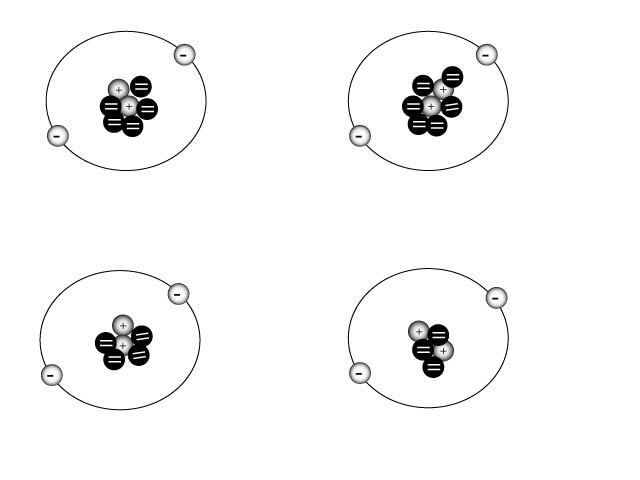 All of the diagrams above are an example of a. | helium isotopes | c. | Helium ions | b. | carbon atoms | d. | hydrogen ions |
|
|
|
15.
|
Atomic number represents the number of
a. | gluons | c. | quarks | b. | protons | d. | neutrons |
|
|
|
16.
|
An electron has a
a. | negative charge | c. | positive charge | b. | neutral charge |
|
|
|
17.
|
If a stable atom has 3 protons and 4 neutrons how many electrons will it
have?
|


